I thought i would share this with you.
This is my anodising power supply.
This is a highly accurate laboratory grade power supply, manufactured by Elektro Automatik which i couldnt recommend highly enough.
There are several different models in the 6000 range, offering different voltages and currents.
The model i have, is the EA-PSI 6072-03 which gives me 0-72V and up to 3A.
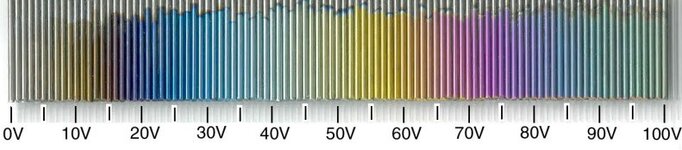
This is great for anodising moderate sized pieces of aluminium, it could easily handle the surface area of a few pens at the same time. The problem however, is that it only goes upto 72V. for anodising aluminium this isnt an issue as i rarely go past 18V. however with the colour spectrum of titanium going up to around 110V's, there are some colours i simply cant reach. how often would i want pink or green? well thats not the point it still winds me up :biggrin: With this in mind i intend on buying thePSI 6150-01 too, which will give me 0 - 150V @ 1.2A which is perfect for titanium. Needless to say i would strictly use this PSU for titanium work, and the other for anodising.
so other than getting these useful voltages... whats so good about it?
Well, other than getting pin point accuracy which you really need if you want a specific colour anodising titanium, and avoiding many headaches, they have some advanced bells and whistles:
CC & CV
These are modes you will commonly find on a good power supply, and it stands for Constant Current, & Constant voltage. Let me give you an example of why this is a useful feature.
The electrolyte which you anodise in, conducts electricity far more efficiently when it is warmer. so much so that when your acid bath heats up a fair bit, say half ay through your process, its such an efficient conductor it can burn and pit your piece of aluminium. While the solution is cold, its a poor conductor, meaning its barely even creating an oxidised layer on your piece because there is not enough current flowing through it - Despite that you calculated you needed 1.2A and set the power supply to 1.2A, to anodise the surface area of the piece of aluminium you have in the bath. If you were to use a ammeter, you would see its not actually using 1.2A, because the solution hasn't warmed up yet and its conducting poorly.
So what do we do other than wait for the solution to warm up? well, we crank up the voltage. Turning up the voltage gives it more power to push its way through the poor conducting electrolyte, so it can in fact then deliver the 1.2A as you were intending. this may be that instead of using the expected 12Volts, you are now driving it at 18V so it can deliver the 1.2A. Great! so we are now actually getting a good anodised layer.... for now....
the draw back here, is that we are creating more heat. Like i said earlier, a warmer solution will conduct more efficiently. so... now we have too many volts flowing through it! infact we only need for example, 16V's to deliver the 1.2A ... lets say five minutes went by before we realised this... you could have just spoiled the surface of the anodised layer with pitting. so now we turn the voltage down until it can give the 1.2A we were after..
Some more time passes, the solution warms up, and now it only needs 14V ... and so on and so forth.
When you set your power supply in CC mode, you tell it to maintain a current of 1.2A. It now goes into auto pilot mode and adjusts the voltage for you instantly, giving it just enough voltage that it needs. As the electrolyte warms up, it turns the voltage down for you.
So in summary, CC gives you an even, near perfect anodised layer from the very start of your process, right til the very end. Weheras before you may have been running your system for an hour, but only actually creating an optimal layer for 20 minutes of that time.
Constant voltage isnt alot of use to us in anodising aluminium, but its fantastic for anodising titanium! it behaves in the same manner as CC, but varying the current to provide a stable voltage. As its voltages which provide us with certain colours with titanium, its that value we want to lock on to.
Presets
if you are doing the same style of pen over and over you will know how much current you will need for the job etc.. then why not set each pen style as a preset. then your good to go at the push of a button
What else? RS232
This allows me to control the power supply by the workshop computer instead of the buttons on the front. I find this easier than fiddling with the buttons on the front, having to press them in the right order to get the setting you want etc. It also gives you greater control with real time read outs on your computer screen, and a plot graph showing how it has performed and what it has delivered to your tank throughout the entire process.
Timers can be set on the software telling the power supply to run for an hour etc, voltage sweeps can be set to cascade through different voltages.. good for creating rainbow effects on titanium
These power supplies are not cheap.... but then again.. neither was your lathe.
Expect to pay in the region of £400 for these ($645)
(now to make you a little bit sick.. i paid £50.00 ...... because a courier cracked the plastic 10mm on the top of the bezel.. A dab of CA glue and its good as new... can barely see the crack line )
For those which are interested, theres a full spec sheet here which lists the specs of each of the models too.
http://www.elektroautomatik.de/fileadmin/pdf/datasheets/datasheet_psi6000.pdf